Chronic myelomonocytic leukemia (CMML) is a rare hematologic cancer characterized by sustained monocytosis, bone marrow dysplasia, and features of both myelodysplastic and myeloproliferative disorders. It affects blood cell production and may progress to acute leukemia.
Introduction
Chronic Myelomonocytic Leukemia (CMML) is a rare haematologic malignancy that occupies a unique position at the intersection of myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPN). Distinguished by persistent monocytosis and dysplastic hematopoiesis, CMML presents significant diagnostic and therapeutic challenges.
Epidemiology
Prevalence and Demographics
CMML is an uncommon malignancy, accounting for approximately 1 to 3 cases per 100,000 persons annually. It represents 10 to 15% of all cases of MDS and MPN. The disease predominantly affects older adults, with a median age at diagnosis between 65 and 75 years. There is a slight male predominance, with a male-to-female ratio of about 1.5:1. The incidence increases with age, and paediatric cases are exceedingly rare.
Risk Factors
Known risk factors for CMML include advanced age, male gender, prior exposure to cytotoxic chemotherapy or radiation therapy, and certain inherited genetic syndromes, such as neurofibromatosis type 1. A family history of haematologic malignancies may also confer an increased risk. However, most cases are sporadic, and definitive environmental or lifestyle risk factors have not been established.
Pathophysiology
Disease Mechanisms
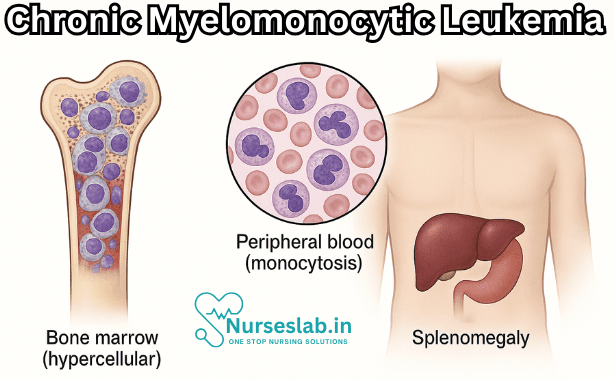
CMML is characterised by clonal proliferation of myeloid cells, particularly monocytes, in the bone marrow and peripheral blood. The pathogenesis involves dysregulation of haematopoietic stem cell differentiation, resulting in both increased production and impaired maturation of monocytic and granulocytic lineages. The bone marrow typically demonstrates hypercellularity, dysplasia of one or more cell lines, and increased monocyte precursors.
Genetic Mutations
Recent advances in molecular genetics have identified several recurrent somatic mutations in CMML. The most frequently mutated genes include TET2 (seen in 40–60% of cases), SRSF2, ASXL1, and RUNX1. Mutations in the RAS pathway (NRAS, KRAS, CBL) are also implicated, particularly in proliferative forms of the disease. These genetic alterations contribute to aberrant cell proliferation, impaired apoptosis, and resistance to therapy. The presence and combination of specific mutations influence disease phenotype, prognosis, and response to treatment.
Clinical Presentation
Signs and Symptoms
CMML may present insidiously, with symptoms often attributed to cytopenias or organ infiltration. The most common clinical features include:
- Fatigue and weakness (secondary to anaemia)
- Fever and night sweats
- Unintentional weight loss
- Easy bruising or bleeding (due to thrombocytopenia)
- Recurrent infections (related to neutropenia)
- Splenomegaly and, less commonly, hepatomegaly
- Bone pain or arthralgia
Some patients remain asymptomatic and are diagnosed incidentally during routine blood tests. The disease course is variable, with some individuals experiencing indolent progression and others developing aggressive features or transformation to acute myeloid leukaemia (AML).
Disease Progression
CMML is a heterogeneous disorder, and its progression is influenced by clinical, cytogenetic, and molecular factors. The transformation to AML occurs in approximately 15–30% of cases, heralded by a rapid increase in blast cells, worsening cytopenias, and refractory symptoms.
Diagnosis
Diagnostic Criteria
The diagnosis of CMML is established based on the World Health Organization (WHO) criteria, which require:
- Persistent monocytosis in peripheral blood (>1 × 109/L, >10% of white blood cells)
- Dysplasia in one or more myeloid cell lines in bone marrow
- Exclusion of other causes of monocytosis, such as infections or inflammatory disorders
- Blast count <20% in peripheral blood and bone marrow (to distinguish from AML)
- Absence of BCR-ABL1 fusion gene (to rule out chronic myeloid leukaemia)
Laboratory Tests
Standard laboratory investigations include:
- Complete blood count (CBC) demonstrating monocytosis, anaemia, and/or thrombocytopenia
- Peripheral blood smear showing dysplastic monocytes and granulocytes
- Bone marrow aspiration and biopsy for cellularity, morphology, and blast count
- Cytogenetic analysis for chromosomal aberrations (e.g., trisomy 8, monosomy 7)
- Molecular testing for gene mutations (e.g., TET2, ASXL1, SRSF2)
Imaging
Imaging studies, such as abdominal ultrasound or computed tomography (CT), may be employed to assess splenomegaly or hepatomegaly. Positron emission tomography (PET) is not routinely indicated unless extramedullary disease is suspected.
Classification
Subtypes and Staging
The WHO classifies CMML into two major subtypes based on blast percentage:
- CMML-1: <5% blasts in peripheral blood and <10% blasts in bone marrow
- CMML-2: 5–19% blasts in peripheral blood or 10–19% in bone marrow
The distinction is clinically meaningful, as CMML-2 is associated with a higher risk of progression to AML and poorer prognosis.
Further subclassification is based on the white blood cell count:
- Myeloproliferative CMML (MP-CMML): Total WBC >13 × 109/L
- Myelodysplastic CMML (MD-CMML): Total WBC ≤13 × 109/L
This stratification guides therapeutic decisions and enables risk-adapted management.
Treatment Options
Chemotherapy
Cytotoxic chemotherapy is primarily reserved for patients with advanced disease, high blast counts, or those progressing to AML. Hypomethylating agents, such as azacitidine and decitabine, are the mainstay of therapy and have demonstrated efficacy in improving haematologic parameters and delaying disease progression. Conventional cytotoxic agents, such as cytarabine and anthracyclines, may be employed in selected cases, particularly during AML transformation.
Targeted Therapy
The advent of targeted therapies has revolutionised CMML management. Agents targeting the RAS pathway (e.g., MEK inhibitors), JAK2 inhibitors, and drugs modulating epigenetic regulators are under active investigation. Allogeneic stem cell transplantation remains the only potentially curative option, particularly for younger patients or those with high-risk disease. However, its applicability is limited by age, comorbidities, and donor availability.
Stem Cell Transplantation
Allogeneic haematopoietic stem cell transplantation (HSCT) is recommended for selected patients with adverse prognostic features, CMML-2, or those who progress to AML. Conditioning regimens vary from myeloablative to reduced-intensity protocols. Transplant-related morbidity and mortality, risk of graft-versus-host disease (GVHD), and post-transplant relapse must be considered when evaluating candidacy for HSCT.
Supportive Care
Supportive care is crucial in CMML management and includes transfusion support, management of infections, iron chelation therapy for transfusion-related iron overload, and symptomatic treatment of cytopenias. Growth factors (e.g., granulocyte colony-stimulating factor) may be used judiciously in cases of severe neutropenia.
Experimental and Investigational Therapies
Emerging therapies, such as immune checkpoint inhibitors, monoclonal antibodies, and small molecule inhibitors targeting specific mutations, are being evaluated in clinical trials. Participation in research studies is encouraged for eligible patients, given the limited efficacy of existing treatments.
Prognosis
Survival Rates
The prognosis of CMML varies widely and is influenced by disease subtype, cytogenetic abnormalities, molecular mutations, and patient comorbidities. Median overall survival ranges from 20 to 36 months, with CMML-1 generally associated with better outcomes than CMML-2. Transformation to AML portends a poor prognosis, with median survival dropping to less than 6 months.
Factors Affecting Outcomes
Adverse prognostic factors include advanced age, high blast counts, myeloproliferative phenotype, presence of ASXL1 or RUNX1 mutations, and unfavourable cytogenetics (e.g., monosomy 7). Several prognostic scoring systems, such as the CMML-specific Prognostic Scoring System (CPSS), incorporate clinical, cytogenetic, and molecular data to stratify risk and guide management.
Patient Management
Monitoring and Follow-up
Optimal management of CMML requires regular monitoring of haematologic parameters, assessment of disease progression, and surveillance for treatment-related complications. Follow-up intervals depend on disease stability and treatment modality. Bone marrow evaluation, cytogenetic studies, and molecular testing are integral to tracking response and detecting early relapse.
Quality of Life
Given the chronic nature of CMML and the burden of cytopenias, supportive care to address anaemia, infection risk, and symptom management is essential. Psychological support, social services, and patient education contribute to improved quality of life. Advance care planning and palliative interventions should be considered for patients with refractory or progressive disease.
Nursing Care of Patients with Chronic Myelomonocytic Leukemia
The complexities of CMML require a nuanced and holistic nursing approach, which addresses both the physiological and psychological challenges the patient may face.
Initial Nursing Assessment
A thorough nursing assessment is foundational for optimal care. Key areas include:
- History and Physical Examination: Assess for symptoms such as fatigue, fever, night sweats, weight loss, infections, and bleeding. Document the patient’s medical history, prior treatments, comorbidities, and baseline functional status.
- Laboratory and Diagnostic Studies: Monitor complete blood counts (CBC), differential, bone marrow biopsy results, cytogenetic studies, and markers of organ function.
- Psychosocial Assessment: Evaluate the patient’s emotional response, coping mechanisms, social support systems, and understanding of the disease.
Key Nursing Diagnoses
Several nursing diagnoses may be relevant for patients with CMML, including:
- Risk for infection related to leukopenia and immunosuppression
- Risk for bleeding related to thrombocytopenia and coagulopathies
- Fatigue related to anemia and treatment side effects
- Impaired comfort related to disease progression and treatment
- Impaired nutrition related to decreased intake and metabolic demands
- Disturbed body image and anxiety related to disease impact and therapy
Planning and Implementing Nursing Interventions
Effective nursing interventions require an individualized plan that incorporates the patient’s unique needs, disease stage, and preferences.
1. Infection Prevention and Control
Patients with CMML face significant infection risk due to neutropenia and immunosuppression. Strategies include:
- Implement strict hand hygiene protocols for staff, patients, and visitors.
- Monitor for signs and symptoms of infection: fever, chills, cough, sore throat, urinary complaints.
- Promptly report and address any infections; administer antibiotics as prescribed.
- Encourage vaccinations as appropriate, avoiding live vaccines unless approved by the oncology team.
- Educate the patient and family on infection prevention measures—avoiding crowds, sick contacts, and contact with pets or raw foods that may harbor pathogens.
- Maintain a clean environment and ensure proper aseptic techniques during procedures.
2. Bleeding Risk Management
Thrombocytopenia and coagulopathy are common in CMML, requiring vigilance:
- Monitor for signs of bleeding—petechiae, ecchymosis, nosebleeds, hematemesis, melena.
- Assess platelet counts regularly and report critical drops promptly.
- Minimize invasive procedures when possible; use soft toothbrushes and electric razors.
- Educate patient and family about avoiding activities that could lead to trauma or injury.
- Administer platelet transfusions as ordered and monitor for transfusion reactions.
3. Fatigue Management
Fatigue can be profound, impacting quality of life:
- Encourage energy conservation and prioritize activities.
- Assist with activities of daily living (ADLs) as needed.
- Promote rest periods and ensure adequate sleep hygiene.
- Support gentle exercise routines as tolerated to improve stamina and mood.
- Monitor for depression and anxiety, referring for psychological support when indicated.
4. Nutritional Support
Nutrition is pivotal for strength, immune function, and healing:
- Assess nutritional status and consult with a dietitian for individualized plans.
- Recommend small, frequent, nutrient-dense meals to combat anorexia and weight loss.
- Monitor for nausea, vomiting, mucositis, or other GI side effects of therapy, and provide appropriate interventions.
- Encourage adequate hydration and monitor for signs of dehydration.
- Educate on food safety—avoid raw or undercooked foods that may carry infection risk.
5. Symptom Management and Comfort Care
Holistic care addresses pain, discomfort, and psychological distress:
- Assess pain regularly and administer analgesics as prescribed.
- Monitor for side effects of chemotherapy and supportive medications, intervening as necessary.
- Offer complementary therapies such as relaxation techniques, aromatherapy, or gentle massage, as appropriate.
- Provide emotional support, facilitate support groups, and encourage open discussion about fears and concerns.
6. Patient and Family Education
Empowering patients with information can improve coping and adherence:
- Explain the nature of CMML, treatment options, expected side effects, and prognosis in understandable language.
- Provide written materials and reliable online resources.
- Encourage questions and active participation in care decisions.
- Teach self-monitoring for signs of infection, bleeding, or other complications.
- Discuss advance directives and goals of care early in the disease process.
7. Psychosocial and Emotional Support
The emotional impact of CMML is substantial, affecting both patient and family:
- Screen for depression, anxiety, and post-traumatic stress symptoms.
- Facilitate access to counseling, psychiatric care, or pastoral support as desired.
- Involve family members in care planning and provide education tailored to their needs.
- Support patient autonomy, dignity, and decision-making at all stages.
8. End-of-Life Care and Palliative Support
In advanced stages, the goals may shift toward comfort and quality of life:
- Initiate discussions around palliative care early, respecting patient wishes.
- Manage symptoms aggressively—pain, dyspnea, fatigue, anxiety.
- Coordinate with interdisciplinary teams for seamless care transitions (home, hospice, hospital).
- Offer bereavement support to family members following patient death.
Interdisciplinary Collaboration
Care for patients with CMML is best delivered by an interdisciplinary team, including physicians, nurses, social workers, dietitians, pharmacists, and therapists. Nurses play a central role in care coordination, advocacy, and communication between all stakeholders.
Documentation and Evaluation
Ongoing documentation of assessments, interventions, and patient responses is essential to adapt care plans and measure outcomes. Regular evaluation allows for timely modifications in the face of changing clinical status, treatment side effects, or patient preferences.
REFERENCES
- Patnaik MM, Tefferi A. Chronic myelomonocytic leukemia: 2024 update on diagnosis, risk stratification and management. Am J Hematol. 2024 Jun;99(6):1142-1165. https://pubmed.ncbi.nlm.nih.gov/38450850/.
- Geissler K. Molecular Pathogenesis of Chronic Myelomonocytic Leukemia and Potential Molecular Targets for Treatment Approaches. https://pmc.ncbi.nlm.nih.gov/articles/PMC8514979/. Front Oncol. 2021;11:751668.
- Thomopoulos TP, Bouhla A, Papageorgiou SG, Pappa V. Chronic myelomonocytic leukemia – a review. Expert Rev Hematol. 2021 Jan;14(1):59-77. doi: 10.1080/17474086.2021.1860004. Epub 2020 Dec 12. PMID: 33275852.
- Marando L, Csizmar CM, Patnaik MM. Chronic myelomonocytic leukemia: molecular pathogenesis and therapeutic innovations. Haematologica. 2025 Jan 1;110(1):22-36. doi: 10.3324/haematol.2024.286061. PMID: 39415698; PMCID: PMC11694134.
- Xu R, Li M, Wu P, et al. Hypomethylating agents in the treatment of chronic myelomonocytic leukemia: a meta-analysis and systematic review. https://pubmed.ncbi.nlm.nih.gov/33706667/. Hematology. 2021;26(1):312-320.
- Patnaik MM. How I diagnose and treat chronic myelomonocytic leukemia. Haematologica. 2022 Jul 1;107(7):1503-1517. doi: 10.3324/haematol.2021.279500. PMID: 35236051; PMCID: PMC9244829.
Stories are the threads that bind us; through them, we understand each other, grow, and heal.
JOHN NOORD
Connect with “Nurses Lab Editorial Team”
I hope you found this information helpful. Do you have any questions or comments? Kindly write in comments section. Subscribe the Blog with your email so you can stay updated on upcoming events and the latest articles.





