Explore antigen-antibody reactions: precise immune interactions where antibodies recognize and bind to antigens. These reactions underpin diagnostic tests, vaccine efficacy, and immune defense mechanisms—critical for nursing, microbiology, and public health applications in infection control and disease management.
Introduction
Antigen-antibody reactions are fundamental to immunology and clinical diagnostics. These interactions underpin the body’s ability to recognise and respond to foreign substances, making them central to both natural immunity and laboratory assays. From the earliest serological tests to today’s highly sensitive multiplex platforms, antigen-antibody reactions have evolved to enable precise disease detection, monitoring, and research.
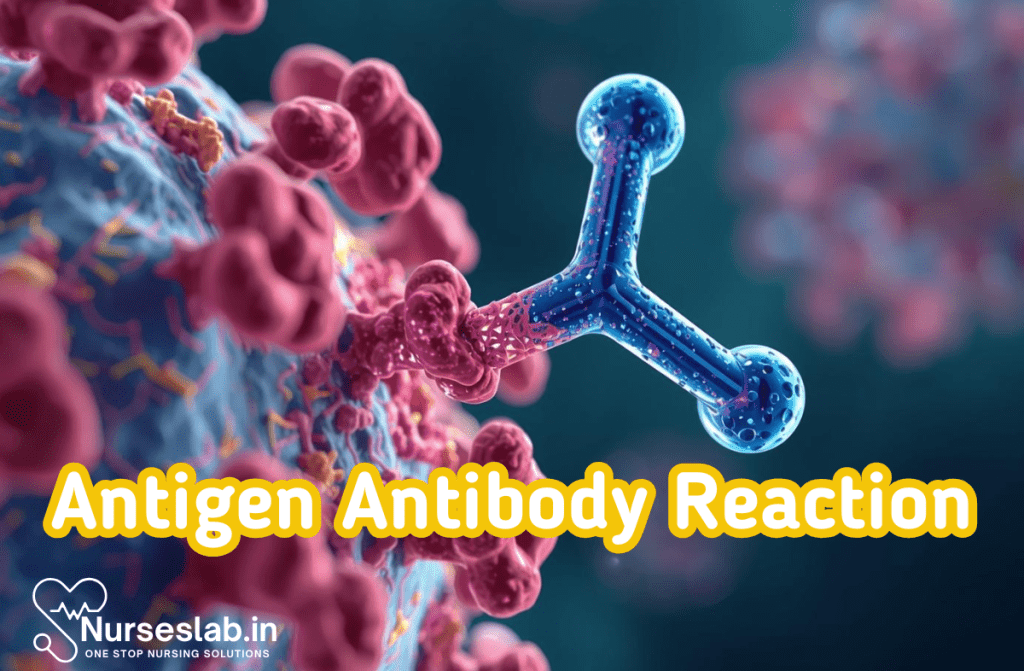
Historical Context and Significance
The study of antigen-antibody reactions began in the late 19th and early 20th centuries with the discovery of antibodies and their role in immunity. Early serological tests such as precipitation and agglutination laid the foundation for modern immunoassays. Over time, technological advances have expanded the scope of immunoassays, enabling the detection of minute quantities of antigens and antibodies, thus transforming diagnostics, research, and therapeutic monitoring.
Basic Principles of Antigen-Antibody Reactions
Structure of Antigens and Antibodies
Antigens are molecules, typically proteins or polysaccharides, that elicit an immune response. They possess specific regions called epitopes, which are recognised by antibodies. Antibodies, or immunoglobulins, are Y-shaped glycoproteins produced by B lymphocytes. Each antibody has two antigen-binding sites formed by variable regions of its heavy and light chains. This structural complementarity underlies the specificity of antigen-antibody interactions.
Binding Mechanisms
Antigen-antibody binding is a non-covalent interaction involving hydrogen bonds, electrostatic forces, van der Waals forces, and hydrophobic interactions. The unique fit between an antibody’s paratope and the antigen’s epitope determines the reaction’s specificity.
Specificity, Affinity, and Avidity
Specificity refers to the ability of an antibody to bind exclusively to a particular antigen. Affinity describes the strength of binding between a single antigenic epitope and an antibody’s paratope. Avidity is the overall strength of binding when multiple antigen-antibody interactions occur simultaneously, as seen with multivalent antigens and antibodies.
Types of Antigen-Antibody Reactions
Precipitation
Precipitation occurs when soluble antigen and antibody interact to form an insoluble complex. This reaction is the basis of several laboratory techniques, including immunodiffusion and immunoelectrophoresis. The visible precipitate forms at the optimal antigen-antibody ratio, known as the equivalence zone.
Agglutination
Agglutination involves the clumping of particulate antigens (such as cells or latex beads) when specific antibodies bind to them. This reaction is widely used in blood typing and rapid diagnostic tests for infectious agents.
Complement Fixation
In complement fixation, antigen-antibody complexes activate the complement system, leading to cell lysis or other immune responses. This reaction is utilised in the complement fixation test (CFT) for detecting antibodies against pathogens.
Neutralisation
Neutralisation refers to the ability of antibodies to block the biological activity of toxins or viruses, rendering them harmless. This principle is harnessed in neutralisation assays to assess protective immunity.
Opsonisation
Opsonisation is the process by which antibodies coat pathogens, enhancing their uptake and destruction by phagocytic cells. This reaction is vital for immune defence and is studied in functional immunology assays.
Other Reaction Types
Additional antigen-antibody reactions include immunofluorescence, chemiluminescence, and enzyme-linked reactions, which form the basis of advanced immunoassays discussed later in this article.
Conventional Immunoassays
Overview
Conventional immunoassays leverage antigen-antibody reactions to detect and quantify specific molecules in clinical specimens. These assays are foundational in medical diagnostics and research.
Radioimmunoassay (RIA)
RIA is a sensitive assay that uses radioactively labelled antigens or antibodies to measure analyte concentrations. The technique involves competitive binding, separation of bound and free fractions, and quantification using a scintillation counter. Despite its high sensitivity, RIA has declined due to concerns over radioactive waste and safety.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA is a versatile, widely used immunoassay based on enzyme-linked antibodies. It involves immobilising antigen or antibody on a solid surface, adding a sample, and detecting bound analyte using an enzyme-substrate reaction that generates a measurable colour change. ELISA formats include direct, indirect, sandwich, and competitive assays, each suited for different applications.
Immunodiffusion
Immunodiffusion techniques, such as Ouchterlony double diffusion, involve the movement of antigens and antibodies through a gel matrix, resulting in visible precipitin lines where they meet at optimal concentrations. These methods are used for qualitative analysis of antigen-antibody specificity.
Immunoelectrophoresis
Immunoelectrophoresis combines electrophoretic separation of antigens with immunodiffusion, allowing simultaneous identification and quantification of multiple proteins in complex mixtures. It is particularly useful in serum protein analysis and immunoglobulin profiling.
Newer Immunoassays
Evolution of Immunoassays
Advancements in immunoassay technology have focused on improving sensitivity, specificity, throughput, and automation. Newer assays address limitations of conventional methods and enable multiplex detection of multiple analytes in a single run.
Automation
Modern immunoassay platforms integrate automated sample handling, reagent dispensing, incubation, washing, and detection. Automation improves reproducibility, reduces human error, and increases throughput, making these systems indispensable in clinical laboratories.
Multiplex Assays
Multiplex immunoassays use bead-based or microarray technologies to simultaneously detect multiple antigens or antibodies. These assays are valuable in research, where profiling of cytokines, hormones, or pathogen panels is required.
Biosensors
Biosensor-based immunoassays employ transducers (optical, electrochemical, or piezoelectric) to convert antigen-antibody interactions into measurable signals. These assays offer rapid, point-of-care testing with high sensitivity and specificity.
Enzyme-Linked Fluorescent Assay (ELFA)
Principle
ELFA combines the specificity of immunoassays with the sensitivity of fluorescence detection. The assay utilises enzyme-labelled antibodies or antigens, where the enzymatic reaction produces a fluorescent product upon substrate addition. The intensity of fluorescence correlates with analyte concentration.
Methodology
Typically, ELFA involves immobilising the capture antibody or antigen on a solid phase, adding the specimen, and introducing an enzyme-labelled detection antibody. After incubation and washing, a fluorogenic substrate is added, and the resulting fluorescence is measured using a fluorometer.
Instrumentation
ELFA platforms consist of automated workstations equipped with incubators, washing modules, and sensitive fluorescence detectors. These instruments enable high-throughput testing with minimal manual intervention.
Applications
ELFA is used for the detection of infectious agents, hormones, tumour markers, and allergens. Its high sensitivity makes it suitable for early diagnosis and monitoring of disease progression.
Advantages and Limitations
- High sensitivity and specificity
- Wide dynamic range
- Automatable and suitable for high-throughput
- Requires specialised instrumentation and reagents
- Potential for background fluorescence and interference
Immunofluorescent Assay (IFA)
Principle
IFA detects antigen-antibody interactions using fluorescently labelled antibodies. When these antibodies bind to their target antigens, the complex emits light upon excitation with a specific wavelength, which is visualised under a fluorescence microscope.
Direct and Indirect Methods
Direct IFA involves the use of a fluorescently labelled antibody that binds directly to the target antigen. Indirect IFA uses an unlabelled primary antibody followed by a fluorescently labelled secondary antibody that recognises the primary antibody, increasing sensitivity.
Protocols
Samples (cells, tissues, or slides) are incubated with the appropriate antibodies, washed to remove unbound reagents, and mounted for microscopic examination. Positive reactions are indicated by distinct fluorescence patterns.
Clinical Uses
- Detection of viral, bacterial, and parasitic infections
- Autoantibody screening in autoimmune diseases
- Identification of tumour markers
- Allergy testing
Interpretation
Interpretation requires expertise in recognising fluorescence patterns and distinguishing specific signals from background noise. Controls are essential for accurate results.
Chemiluminescence Immunoassay (CLIA)
Principle
CLIA is based on the emission of light during a chemical reaction, which occurs when an enzyme-labelled antibody or antigen reacts with a chemiluminescent substrate. The emitted light is directly proportional to the amount of analyte present and is measured by a luminometer.
Reagents
CLIA reagents include enzyme-labelled antibodies or antigens, chemiluminescent substrates (such as luminol or acridinium esters), buffers, and wash solutions. These reagents are optimised for stability and signal strength.
Instrumentation
Automated CLIA analysers perform sample processing, incubation, washing, and signal measurement. The luminometer detects and quantifies the emitted light, enabling rapid and sensitive analysis.
Workflow
Samples are incubated with labelled antibodies, washed to remove unbound components, and exposed to the chemiluminescent substrate. The resulting light emission is measured, and the analyte concentration is calculated based on standard curves.
Clinical Relevance
CLIA is extensively used for hormone assays, infectious disease serology, tumour marker detection, and therapeutic drug monitoring. Its high sensitivity, specificity, and rapid turnaround make it preferred in many diagnostic laboratories.
Comparison with Other Assays
- Higher sensitivity and dynamic range than ELISA
- Faster processing and reduced background noise
- Requires specialised instrumentation and reagents
Comparative Analysis of Immunoassays
Strengths and Weaknesses
| Assay | Strengths | Limitations |
| RIA | Very high sensitivity, quantitative | Radioactive hazards, disposal issues |
| ELISA | Versatile, automatable, cost-effective | Moderate sensitivity, potential for cross-reactivity |
| ELFA | High sensitivity, fluorescence-based, automatable | Expensive instrumentation, background fluorescence |
| IFA | Visualisation of antigen distribution, high specificity | Subjective interpretation, labour-intensive |
| CLIA | Highest sensitivity, rapid, wide dynamic range | Costly equipment and reagents |
| Multiplex | Simultaneous detection, high throughput | Complex data analysis, expensive platforms |
Selection Criteria for Clinical and Research Use
Selection of an immunoassay depends on factors such as required sensitivity, specificity, throughput, available instrumentation, cost, and sample type. For routine diagnostics, ELISA and CLIA are preferred for their balance of sensitivity and automation. Research applications may benefit from multiplex and biosensor-based assays for comprehensive profiling.
Applications in Diagnostics and Research
Infectious Diseases
Immunoassays are pivotal in diagnosing bacterial, viral, and parasitic infections. ELISA and CLIA are commonly used for HIV, hepatitis, dengue, and COVID-19 serology. IFA is valuable for detecting intracellular pathogens and visualising localisation in tissues.
Autoimmune Disorders
Detection of autoantibodies using ELISA, CLIA, and IFA aids in the diagnosis of conditions such as systemic lupus erythematosus, rheumatoid arthritis, and autoimmune thyroiditis. Multiplex assays enable simultaneous screening for multiple autoantibodies.
Oncology
Tumour markers (e.g., PSA, CEA, AFP) are measured using immunoassays for cancer screening, diagnosis, and monitoring. CLIA and ELFA offer high sensitivity for early detection and therapeutic monitoring.
Allergy Testing
Immunoassays detect allergen-specific IgE antibodies, facilitating diagnosis and management of allergic diseases. Multiplex platforms allow comprehensive allergen profiling.
Other Applications
Immunoassays are used in hormone quantification (e.g., thyroid, reproductive hormones), drug monitoring, vaccine efficacy studies, and basic immunological research.
Future Directions in Immunoassay Development
Emerging Technologies
Innovations such as microfluidics, nanotechnology, and digital immunoassays are enhancing sensitivity, speed, and portability. Point-of-care devices and wearable biosensors promise decentralised testing and real-time monitoring.
Trends and Potential Improvements
Trends include integration with artificial intelligence for data interpretation, development of universal platforms, and reduction in assay time. Improvements in reagent stability, miniaturisation, and multiplexing will further expand clinical and research applications.
Conclusion
Antigen-antibody reactions form the cornerstone of immunology and clinical diagnostics. The development of diverse immunoassays—from conventional ELISA and RIA to advanced ELFA, IFA, and CLIA—has revolutionised disease detection, monitoring, and research. Understanding the principles, strengths, and limitations of each assay is essential for selecting appropriate methodologies in clinical and research settings. As technology advances, immunoassays will continue to evolve, offering greater sensitivity, automation, and accessibility, ultimately improving patient care and advancing medical science.
REFERENCES
- Apurba S Sastry, Essential Applied Microbiology for Nurses including Infection Control and Safety, First Edition 2022, Jaypee Publishers, ISBN: 978-9354659386
- Joanne Willey, Prescott’s Microbiology, 11th Edition, 2019, Innox Publishers, ASIN- B0FM8CVYL4.
- Anju Dhir, Textbook of Applied Microbiology including Infection Control and Safety, 2nd Edition, December 2022, CBS Publishers and Distributors, ISBN: 978-9390619450
- Gerard J. Tortora, Microbiology: An Introduction 13th Edition, 2019, Published by Pearson, ISBN: 978-0134688640
- Durrant RJ, Doig AK, Buxton RL, Fenn JP. Microbiology Education in Nursing Practice. J Microbiol Biol Educ. 2017 Sep 1;18(2):18.2.43. https://pmc.ncbi.nlm.nih.gov/articles/PMC5577971/
Stories are the threads that bind us; through them, we understand each other, grow, and heal.
JOHN NOORD
Connect with “Nurses Lab Editorial Team”
I hope you found this information helpful. Do you have any questions or comments? Kindly write in comments section. Subscribe the Blog with your email so you can stay updated on upcoming events and the latest articles.





