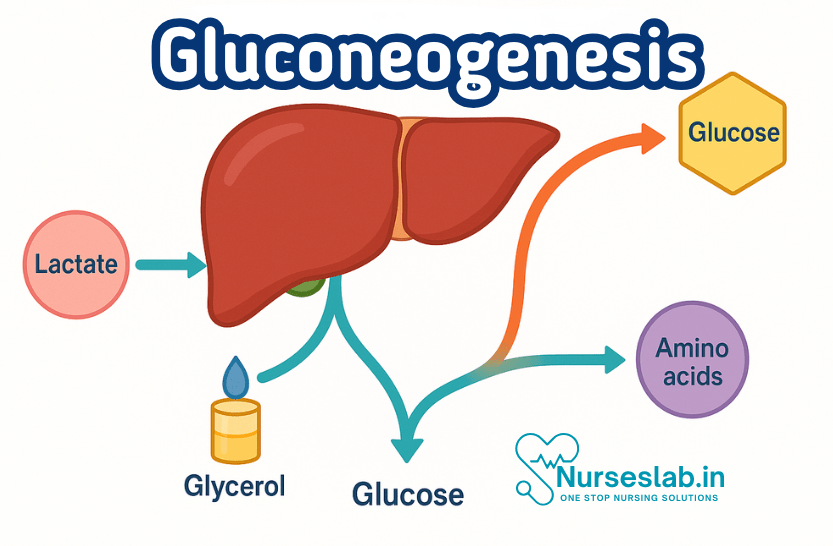
Gluconeogenesis is the biochemical process of synthesizing glucose from non-carbohydrate precursors such as amino acids, lactate, and glycerol. Occurring mainly in the liver and kidneys, it maintains blood glucose levels during fasting, exercise, or low-carbohydrate intake.
What is Gluconeogenesis?
Definition of Gluconeogenesis
Gluconeogenesis is the process by which glucose is synthesised from non-carbohydrate precursors. Unlike glycolysis, which breaks down glucose to generate energy, gluconeogenesis builds glucose molecules, primarily when dietary intake is insufficient or during increased metabolic demand. This pathway is essential for organisms that require a constant supply of glucose, particularly for organs such as the brain, red blood cells, and renal medulla, which rely heavily on glucose as an energy source.
Comparison with Glycolysis
Glycolysis and gluconeogenesis are reciprocally regulated processes. Glycolysis occurs in most cells and converts glucose into pyruvate, producing ATP in the process. Gluconeogenesis, on the other hand, occurs mainly in the liver and kidneys, generating glucose from pyruvate and other substrates. While glycolysis is an energy-yielding pathway, gluconeogenesis is energy-consuming, requiring ATP and GTP to drive the synthesis of glucose.
Overview of Glucose Metabolism
Glucose metabolism encompasses several interconnected pathways: glycolysis, gluconeogenesis, glycogenesis, and glycogenolysis. The balance between these pathways ensures that blood glucose levels remain within a narrow physiological range, preventing both hyperglycaemia and hypoglycaemia. Gluconeogenesis plays a pivotal role during periods when exogenous glucose is unavailable, such as overnight fasting or prolonged physical activity.
Importance of Gluconeogenesis
- The gluconeogenesis cycle is crucial for controlling blood sugar levels during deprivation.
- RBCs, neurons, skeletal muscle, the medulla of the kidney, the testes, and embryonic tissue are just a few of the cells and tissues that need glucose to function.
- Metabolites including lactate, which is created by muscles and RBCs, and glycerol, which is produced by adipose tissue, are removed from the circulation through the Neoglucogenesis cycle.
Biochemical Pathway of Gluconeogenesis
Step-by-Step Pathway
Gluconeogenesis primarily occurs in the cytosol and mitochondria of hepatocytes (liver cells) and, to a lesser extent, in renal cortical cells. The pathway is essentially the reverse of glycolysis, with three key bypass steps to overcome irreversible reactions in glycolysis:
1.Pyruvate to Phosphoenolpyruvate (PEP):
This conversion involves two steps. Pyruvate enters the mitochondria, where pyruvate carboxylase converts it to oxaloacetate using ATP and biotin as a cofactor. Oxaloacetate is then converted to PEP by phosphoenolpyruvate carboxykinase (PEPCK), using GTP.
2.Fructose-1,6-bisphosphate to Fructose-6-phosphate:
The enzyme fructose-1,6-bisphosphatase removes a phosphate group, bypassing the irreversible phosphofructokinase-1 step in glycolysis.
3.Glucose-6-phosphate to Glucose:
Glucose-6-phosphatase dephosphorylates glucose-6-phosphate, allowing free glucose to be released into the bloodstream. This step occurs in the endoplasmic reticulum of liver and kidney cells.
Key Substrates: Lactate, Glycerol, Amino Acids
Gluconeogenesis utilises several non-carbohydrate precursors:
- Lactate: Produced during anaerobic glycolysis, especially in muscle cells. The Cori cycle describes how lactate is transported to the liver, where it is converted back to glucose.
- Glycerol: Released from adipose tissue during lipolysis. Glycerol is converted to dihydroxyacetone phosphate (DHAP), an intermediate in gluconeogenesis.
- Amino Acids: Especially alanine and glutamine, which are transaminated to form pyruvate or other intermediates. This is particularly significant during prolonged fasting or starvation when protein breakdown increases.
Major Organs Involved: Liver and Kidney
The liver is the primary site of gluconeogenesis, responsible for maintaining blood glucose levels during fasting. The kidneys also contribute, particularly during prolonged starvation, accounting for up to 40% of gluconeogenic activity. Both organs possess the necessary enzymes, such as glucose-6-phosphatase, to complete the pathway and release glucose into the circulation.
Key Enzymes and Regulation
Rate-Limiting Enzymes
Three key enzymes regulate the gluconeogenic pathway:
- Pyruvate Carboxylase: Catalyses the conversion of pyruvate to oxaloacetate in the mitochondria. Activated by acetyl-CoA, reflecting increased fatty acid oxidation during fasting.
- Phosphoenolpyruvate Carboxykinase (PEPCK): Converts oxaloacetate to phosphoenolpyruvate. Its expression is tightly regulated by hormonal signals.
- Fructose-1,6-bisphosphatase: Removes a phosphate group from fructose-1,6-bisphosphate, bypassing the rate-limiting step of glycolysis.
- Glucose-6-phosphatase: Hydrolyses glucose-6-phosphate to free glucose, facilitating its release into the bloodstream.
Hormonal Regulation
Gluconeogenesis is finely controlled by hormones in response to metabolic demands:
- Insulin: Released from the pancreas in response to high blood glucose, insulin suppresses gluconeogenesis by inhibiting the expression of gluconeogenic enzymes.
- Glucagon: Secreted during low blood glucose, glucagon stimulates gluconeogenesis by upregulating PEPCK and glucose-6-phosphatase.
- Cortisol: A stress hormone produced by the adrenal cortex, cortisol promotes gluconeogenesis by increasing the transcription of key enzymes, particularly during prolonged stress or illness.
Allosteric Regulation
Apart from hormonal control, gluconeogenic enzymes are subject to allosteric regulation:
- Pyruvate carboxylase is activated by acetyl-CoA, ensuring gluconeogenesis proceeds when energy is supplied by fatty acid oxidation.
- Fructose-1,6-bisphosphatase is inhibited by fructose-2,6-bisphosphate and AMP, preventing gluconeogenesis when energy is abundant.
These regulatory mechanisms ensure that gluconeogenesis operates only when necessary, maintaining metabolic balance.
Physiological Significance
Role During Fasting, Starvation, Exercise, and Stress
Gluconeogenesis is indispensable for survival during periods of limited carbohydrate intake. During overnight fasting, the liver utilises lactate, glycerol, and amino acids to maintain blood glucose. In prolonged starvation, muscle protein breakdown provides amino acids for gluconeogenesis, while the kidneys increase their contribution to glucose production. During intense exercise, lactate produced by muscles is recycled via the Cori cycle. Stress and illness, mediated by cortisol, further increase gluconeogenic activity to meet increased energy demands.
Maintaining Blood Glucose Levels
Glucose is the primary energy source for the brain, erythrocytes, and certain other tissues. The body’s ability to maintain glucose homeostasis is critical, as both hypoglycaemia and hyperglycaemia can have serious consequences. Gluconeogenesis provides a continuous supply of glucose, particularly when glycogen stores are depleted, safeguarding vital organs and functions.
Clinical Relevance
Disorders Related to Gluconeogenesis
Several clinical conditions arise from disruptions in gluconeogenesis:
- Diabetes Mellitus: In type 2 diabetes, insulin resistance impairs the suppression of gluconeogenesis, leading to elevated blood glucose levels. Understanding this mechanism is crucial for managing hyperglycaemia and tailoring therapy.
- Hypoglycaemia: Defective gluconeogenesis, as seen in certain inborn errors or liver dysfunction, can result in dangerously low blood glucose, manifesting as confusion, seizures, or coma.
- Inborn Errors of Metabolism: Genetic deficiencies in enzymes such as glucose-6-phosphatase (e.g., von Gierke disease) impair gluconeogenesis, leading to hypoglycaemia and metabolic acidosis.
- Liver and Kidney Diseases: Hepatic or renal impairment can reduce gluconeogenic capacity, affecting glucose homeostasis, especially during stress or fasting.
Diagnostic Markers
Laboratory tests play a pivotal role in assessing gluconeogenic function:
- Blood glucose levels, especially during fasting, can indicate impaired gluconeogenesis.
- Lactate and pyruvate concentrations help diagnose disorders of carbohydrate metabolism, including lactic acidosis.
- Enzyme assays and genetic testing may identify inborn errors affecting gluconeogenic enzymes.
Implications for Nursing Practice
Monitoring Patients
Nurses are often at the forefront of patient monitoring. Understanding gluconeogenesis allows nurses to interpret blood glucose readings, recognise signs of metabolic imbalance, and promptly address complications. For example, during critical illness or post-surgery, increased gluconeogenesis may contribute to hyperglycaemia, necessitating vigilant monitoring and adjustment of insulin therapy.
Understanding Laboratory Results
Interpreting laboratory data is a key nursing responsibility. Abnormal fasting blood glucose, elevated lactate, or changes in liver and kidney function tests may indicate disruptions in gluconeogenesis. Nurses should be able to correlate these findings with clinical presentation and communicate concerns to the multidisciplinary team.
Patient Education
Educating patients and families about the importance of glucose regulation is integral to nursing care. Nurses can explain the role of diet, exercise, and medication in maintaining blood glucose levels, emphasising the significance of gluconeogenesis during fasting or illness. For diabetic patients, understanding why blood glucose rises despite fasting can aid adherence to therapy and dietary recommendations.
Nutritional Considerations
Nutrition profoundly influences gluconeogenic activity. Nurses should advocate for balanced diets, particularly in vulnerable populations such as children, the elderly, and those with chronic illness. Recognising the signs of malnutrition or excessive protein breakdown enables early intervention, preventing complications related to impaired gluconeogenesis.
Summary and Key Takeaways
Gluconeogenesis is a central metabolic pathway, enabling the synthesis of glucose from non-carbohydrate sources, particularly during fasting, stress, or illness. Its regulation involves complex hormonal and allosteric mechanisms, ensuring metabolic balance. Disruptions in gluconeogenesis underlie several clinical conditions, including diabetes, hypoglycaemia, and inborn errors of metabolism. For nurses, a solid grasp of gluconeogenesis enhances patient monitoring, interpretation of laboratory results, and effective patient education. Ultimately, integrating biochemistry into nursing practice fosters better patient outcomes and more informed, holistic care.
REFERENCES
- Harbans Lal, Textbook of Applied Biochemistry and Nutrition& Dietetics 2nd Edition ,November 2024, CBS Publishers and Distributors, ISBN: 978-9394525757
- Suresh K Sharma, Textbook of Biochemistry and Biophysics for Nurses, 2nd Edition, September 2022, Jaypee Publishers, ISBN: 978-9354655760
- Peter J Kennelly, Harpers Illustrated Biochemistry Standard Edition, September 2022, McGraw Hill Lange Publishers, ISBN: 978-1264795673
- Denise R Ferrier, Ritu Singh, Lippincott Illustrated Reviews Biochemistry, Second Edition, June 2024, ISBN- 978-8197055973
- Yadav, Tapeshwar & Bhadeshwar, Sushma. (2022). Essential Textbook of Biochemistry for Nursing.
- Applied Sciences, Importance of Biochemistry for Nursing Practice, November 2, 2023, https://bns.institute/applied-sciences/importance-biochemistry-nursing-practice/
Stories are the threads that bind us; through them, we understand each other, grow, and heal.
JOHN NOORD
Connect with “Nurses Lab Editorial Team”
I hope you found this information helpful. Do you have any questions or comments? Kindly write in comments section. Subscribe the Blog with your email so you can stay updated on upcoming events and the latest articles.





