Choroid Plexus Carcinoma (CPC) is a rare, aggressive brain tumor arising from the choroid plexus, most commonly in children. Understanding its symptoms, imaging findings, diagnosis, and treatment is essential in neurology, oncology, and clinical practice.
Introduction
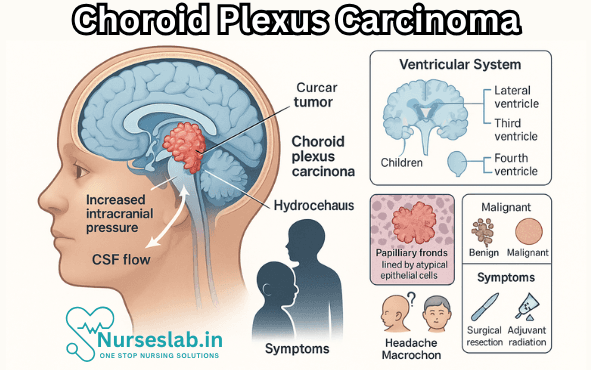
Choroid plexus carcinoma (CPC) is a rare and highly malignant neoplasm arising from the choroid plexus epithelium within the ventricular system of the brain. Although it constitutes a small fraction of all brain tumours, its aggressive nature, challenging diagnosis, and limited treatment options make it a significant topic of concern in neuro-oncology. CPC is predominantly seen in the paediatric population, with a notable impact on morbidity and mortality.
Definition and Classification
What is Choroid Plexus Carcinoma?
Choroid plexus carcinoma is a malignant epithelial tumour originating from the choroid plexus, the specialised tissue in the brain ventricles responsible for cerebrospinal fluid (CSF) production. CPC is classified as a World Health Organization (WHO) grade III tumour, indicating its high-grade, aggressive behaviour. It is distinct from choroid plexus papilloma (CPP), a benign counterpart (WHO grade I), and atypical choroid plexus papilloma (WHO grade II), which has intermediate features.
WHO Classification
According to the WHO Classification of Tumours of the Central Nervous System (2021), choroid plexus tumours are divided into:
- Choroid Plexus Papilloma (CPP) – WHO Grade I: Benign, well-differentiated tumours.
- Atypical Choroid Plexus Papilloma (aCPP) – WHO Grade II: Tumours with increased mitotic activity and atypia but lacking overt malignancy.
- Choroid Plexus Carcinoma (CPC) – WHO Grade III: Malignant, poorly differentiated tumours with evidence of invasion and high mitotic index.
Epidemiology
Incidence and Prevalence
CPC is an uncommon tumour, accounting for less than 1% of all brain tumours and approximately 2-5% of paediatric brain tumours. Its annual incidence is estimated at 0.3 cases per 1 lakh children. In adults, CPC is exceedingly rare, with paediatric cases comprising the vast majority.
Age Distribution
CPC predominantly affects children, with the median age of diagnosis being around 3 years. More than 80% of cases are diagnosed in children under the age of 5 years. There is a slight male predominance reported in some studies.
Geographical and Ethnic Distribution
No significant geographical or ethnic predilection has been consistently observed, though some regions report higher prevalence due to referral patterns and diagnostic capabilities.
Risk Factors
While most CPCs are sporadic, certain genetic syndromes, particularly Li-Fraumeni syndrome (associated with germline TP53 mutations), significantly increase the risk. Family history and inherited cancer predisposition syndromes should be carefully evaluated in affected individuals.
Pathophysiology
Origin and Histogenesis
CPC arises from the epithelium of the choroid plexus, which lines the ventricles and is responsible for the production and regulation of CSF. The tumour may develop in any of the brain’s ventricles, but it most commonly originates in the lateral ventricles in children and the fourth ventricle in adults.
Molecular Biology and Genetic Factors
Recent advances have elucidated several molecular pathways involved in CPC pathogenesis:
- TP53 Mutations: Germline or somatic mutations in the TP53 tumour suppressor gene are commonly implicated, especially in patients with Li-Fraumeni syndrome. TP53 mutations are associated with a more aggressive disease course and poorer outcomes.
- Chromosomal Aberrations: Losses of chromosomes 17p and 22q, as well as other complex karyotypic abnormalities, have been reported.
- Other Genetic Factors: Alterations in genes such as hSNF5/INI1 (SMARCB1), although less frequent, have also been described.
The understanding of these molecular features is critical for risk stratification and the development of targeted therapies.
Tumour Biology
CPCs are characterised by rapid growth, high proliferative index, frequent invasion of adjacent brain parenchyma, and a propensity for CSF dissemination, resulting in leptomeningeal spread and metastatic seeding along the neuraxis.
Clinical Presentation
Symptoms and Signs
The clinical presentation of CPC largely depends on the tumour’s location, size, and the degree of CSF obstruction. The most common presenting features include:
- Raised Intracranial Pressure: Due to obstruction of CSF pathways, leading to hydrocephalus. Symptoms include headache, vomiting, irritability, and lethargy.
- Macrocephaly: In infants, head enlargement may be observed due to open cranial sutures.
- Focal Neurological Deficits: Depending on tumour location, patients may exhibit hemiparesis, ataxia, cranial nerve palsies, or visual disturbances.
- Developmental Delay: Chronic increased intracranial pressure and neurological compromise can result in delayed milestones.
- Seizures: Less common, but may occur if there is cortical involvement.
Age of Onset
The majority of CPC cases are diagnosed in early childhood, typically before the age of 5 years. In rare adult cases, the presentation may be subtler and more insidious.
Diagnostic Workup
Imaging Studies
Imaging is crucial for initial assessment and surgical planning:
- Magnetic Resonance Imaging (MRI): The modality of choice. CPCs appear as large, irregular, heterogeneously enhancing masses, often associated with hydrocephalus and peritumoural oedema. They may display areas of necrosis, cystic change, and intratumoural haemorrhage.
- Computed Tomography (CT): Useful in emergency settings. Tumours are typically hyperdense, with possible calcifications and contrast enhancement.
- CSF Analysis: May reveal malignant cells, particularly in cases with leptomeningeal dissemination, though lumbar puncture is not routinely performed due to the risk of herniation in the setting of raised intracranial pressure.
Histopathology
Definitive diagnosis is established by histopathological examination following surgical resection or biopsy. Key features include:
- Highly cellular tumour with papillary, solid, or mixed architecture.
- Marked nuclear atypia, high mitotic activity, and areas of necrosis.
- Evidence of brain parenchymal invasion.
- Immunohistochemical markers: Positive for cytokeratins, S100, transthyretin, and sometimes GFAP (glial fibrillary acidic protein). High Ki-67 proliferation index is typical.
Differential Diagnosis
The differential diagnosis of CPC includes:
- Choroid plexus papilloma (benign and atypical forms)
- Other intraventricular tumours: ependymoma, medulloblastoma, central neurocytoma
- Metastatic carcinoma (rare in children)
A combination of imaging, histopathology, and immunohistochemistry is essential for accurate diagnosis.
Treatment Modalities
Surgical Management
Surgical resection is the cornerstone of CPC management. The primary goal is maximal safe resection, as the extent of tumour removal is the most significant prognostic factor. Complete resection offers the best chance of long-term survival. However, the location, vascularity, and infiltration of surrounding brain tissue often complicate surgery.
Adjuvant Therapy
- Chemotherapy: Due to the high risk of recurrence and dissemination, multi-agent chemotherapy regimens are employed post-operatively, especially in paediatric patients. Common agents include etoposide, vincristine, cyclophosphamide, and carboplatin. Chemotherapy is particularly important in cases of incomplete resection or metastatic disease.
- Radiotherapy: Craniospinal irradiation (CSI) is considered for older children and adults with residual or metastatic disease. In very young children, radiotherapy is used cautiously due to the risk of neurocognitive side effects. Focal radiotherapy may be used in select cases.
Emerging and Investigational Therapies
Recent advances in molecular genetics have prompted the exploration of targeted therapies, though these remain largely experimental. Immunotherapy, including immune checkpoint inhibitors, and molecularly targeted agents against specific genetic alterations, are under investigation. High-dose chemotherapy with autologous stem cell rescue is also being explored for recurrent or refractory cases.
Supportive Care
Management of hydrocephalus, either through external ventricular drainage or ventriculoperitoneal shunting, is often required. Rehabilitation, neurocognitive support, and psychosocial interventions are crucial components of comprehensive care.
Prognosis and Outcomes
Survival Rates
The prognosis of CPC remains guarded, with reported 5-year overall survival rates ranging from 20% to 40%, depending on various factors. Complete surgical resection is the most important determinant of favourable outcome. Children with gross total resection and favourable molecular profiles have better survival rates.
Prognostic Factors
Several factors influence prognosis:
- Extent of surgical resection (gross total versus subtotal)
- Age at diagnosis (older children fare better than infants)
- Presence of metastases at diagnosis
- TP53 mutation status (mutations confer worse prognosis)
- Response to adjuvant therapy
Recurrence and Follow-Up
Recurrence is common, particularly in cases with incomplete resection or dissemination at diagnosis. Long-term surveillance with periodic MRI is recommended. Recurrences may be managed with repeat surgery, chemotherapy, or radiotherapy, depending on individual patient factors.
Nursing Care of Patients with Choroid Plexus Carcinoma
Nurses play a pivotal role in managing these patients, providing direct support, monitoring for complications, and advocating for both the patient and their family. This document outlines the principles of nursing care for patients diagnosed with CPC, emphasizing physical, psychological, and emotional well-being.
Assessment and Initial Management
Nursing care begins with comprehensive assessment:
- Neurological Evaluation: Routine neurological assessments using standardized tools (e.g., Glasgow Coma Scale) to monitor mental status, motor function, cranial nerve integrity, and reflexes. Observe for seizure activity, changes in consciousness, or focal deficits.
- Vital Signs and ICP Monitoring: Closely monitor blood pressure, pulse, respiratory rate, temperature, and oxygen saturation. Be vigilant for bradycardia, hypertension, or irregular respirations indicative of increased ICP.
- Hydration and Nutrition: Assess for nausea, vomiting, and difficulty swallowing. Collaborate with dieticians to ensure adequate caloric and nutrient intake, considering enteral feeding if oral intake is compromised.
- Pain Assessment: Use age-appropriate pain scales. Provide pharmacological and non-pharmacological interventions for pain and discomfort.
- Family History and Psychosocial Assessment: Understand family dynamics, coping strategies, and psychosocial needs to provide targeted emotional support.
Preoperative Nursing Care
Many patients with CPC require neurosurgical intervention for tumor removal and CSF diversion (e.g., ventriculoperitoneal shunt insertion). Preoperative care includes:
- Preparation for Surgery: Educate patient and family about surgical procedures, risks, expected outcomes, and postoperative care. Obtain informed consent and ensure preoperative labs and imaging are completed.
- Skin and Infection Prevention: Bathe patient with antiseptic solutions and maintain strict aseptic technique to reduce infection risk.
- Managing Anxiety: Offer reassurance, answer questions, and introduce relaxation techniques to alleviate preoperative anxiety.
- Medication Administration: Administer prescribed antibiotics, corticosteroids, or anticonvulsants as ordered.
Postoperative Nursing Care
Following surgery, patients require close monitoring and comprehensive care to detect complications early and promote recovery:
- Monitoring for Neurological Deterioration: Conduct frequent neurological checks, observing for new deficits, changes in consciousness, or signs of herniation.
- ICP Management: Position patient with head elevated (30 degrees) to facilitate venous drainage unless contraindicated. Monitor for CSF leaks, shunt malfunction, or infection.
- Wound Care: Inspect surgical site for redness, swelling, discharge, or dehiscence. Maintain sterile dressings and document findings consistently.
- Pain and Comfort: Administer analgesics as ordered and employ comfort measures such as repositioning, gentle massage, or distraction techniques.
- Prevention of Complications:
- Seizures: Maintain seizure precautions, ensure bed rails are padded, and administer anticonvulsants as prescribed.
- Infections: Practice hand hygiene, monitor for fever, and observe for signs of wound or systemic infection.
- Venous Thromboembolism: Encourage passive range of motion, use compression stockings, and promote early mobilization where feasible.
Long-Term Care and Rehabilitation
CPC treatment often includes adjuvant therapies such as radiotherapy and chemotherapy, which present additional challenges:
- Chemotherapy and Radiation Side Effects: Monitor for bone marrow suppression, mucositis, alopecia, nausea, and vomiting. Administer antiemetics, mouth care, and supportive therapies as needed.
- Neurocognitive and Developmental Support: Collaborate with neuropsychologists, physical, occupational, and speech therapists to address deficits and support rehabilitation.
- Endocrine and Growth Monitoring: Assess for hypothalamic-pituitary dysfunction, growth delays, or precocious puberty due to tumor location or treatment.
- Education and School Reintegration: Liaise with educators to facilitate a smooth transition back to school, adapting plans to accommodate learning needs.
- Emotional and Psychosocial Support: Provide counseling resources for patients and families, encourage participation in support groups, and foster resilience and hope.
Palliative and Supportive Care
For patients with advanced CPC or poor prognosis, the focus may shift to comfort care:
- Pain and Symptom Management: Employ multimodal analgesia and manage distressing symptoms such as dyspnea, agitation, or delirium.
- Advance Care Planning: Facilitate discussions about goals of care, wishes for life-sustaining treatment, and end-of-life options.
- Spiritual and Cultural Sensitivity: Respect spiritual beliefs and cultural practices, integrating them into care plans as appropriate.
- Bereavement Support: Offer counseling and follow-up to family members following loss, connecting them with community resources.
Patient and Family Education
Education is a cornerstone of nursing care for CPC:
- Tumor Biology and Treatment Options: Provide information about disease process, prognosis, and available therapies in age-appropriate language.
- Signs and Symptoms of Complications: Teach families to identify red flags such as sudden headache, vomiting, altered consciousness, or wound changes.
- Medication Management: Educate caregivers on proper medication administration, side effect monitoring, and adherence strategies.
- Home Care and Follow-Up: Instruct on wound care, activity restrictions, nutrition, and scheduling follow-up appointments.
Collaboration and Advocacy
Nurses serve as advocates and coordinators, ensuring the patient receives comprehensive and seamless care:
- Interdisciplinary Collaboration: Work with physicians, pharmacists, therapists, and social workers to develop and revise care plans.
- Resource Coordination: Help families access financial support, transportation, and home health services.
- Policy Advocacy: Support policies and research that improve outcomes and access for patients with rare brain tumors.
REFERENCES
- Brain Tumour Research (U.K.). Choroid Plexus Tumours . https://www.braintumourresearch.org/info-support/types-of-brain-tumour/choroid-plexus-tumours.
- National Cancer Institute (U.S.). Choroid Plexus Tumors Diagnosis and Treatment. https://www.cancer.gov/rare-brain-spine-tumor/tumors/choroid-plexus-tumors. Last updated 8/2024.
- Winn HR, ed. Choroid plexus tumors. In: Youmans and Winn Neurological Surgery. 8th ed. Elsevier; 2023. https://www.clinicalkey.com.
- Radiopaedia. Choroid Plexus Carcinoma . https://radiopaedia.org/articles/choroid-plexus-carcinoma. Last revised 6/2024.
- Townsend CM Jr, et al. Neurosurgery. In: Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 21st ed. Elsevier; 2022. https://www.clinicalkey.com.
- Ruiz-Garcia H, Huayllani MT, Incontri D, et al. Intraventricular choroid plexus tumors: clinical characteristics and impact of current management on survival. https://pubmed.ncbi.nlm.nih.gov/32897467/. J Neurooncol. 2020;149(2):283-292.
Stories are the threads that bind us; through them, we understand each other, grow, and heal.
JOHN NOORD
Connect with “Nurses Lab Editorial Team”
I hope you found this information helpful. Do you have any questions or comments? Kindly write in comments section. Subscribe the Blog with your email so you can stay updated on upcoming events and the latest articles.





