Chronic low back pain is one of the most prevalent and debilitating health conditions in the modern world, affecting millions and often proving resistant to conventional therapies. Among the promising new approaches to managing this persistent ailment is ReActiv8, a device-based therapy rooted in the principle of restorative neurostimulation. This document explores the science, clinical evidence, procedure, patient experience, and future perspectives of ReActiv8, shedding light on how this technology is transforming the landscape of chronic low back pain management.
What is ReActiv8?
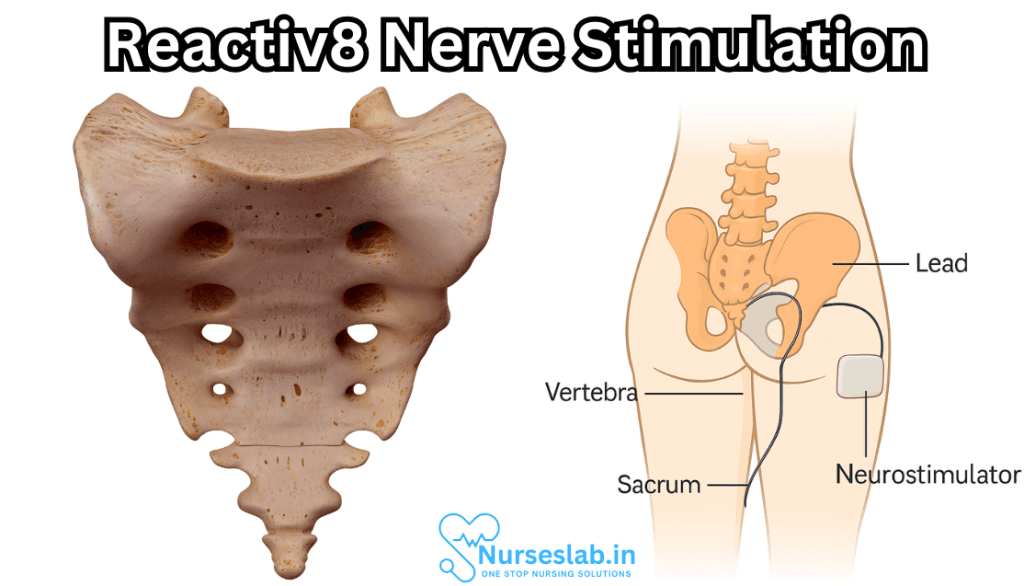
ReActiv8 is a medical device designed to treat chronic low back pain through the technique of restorative neurostimulation. Unlike traditional spinal cord stimulation (SCS), which primarily aims to mask pain signals, ReActiv8 targets the underlying cause of pain by stimulating the nerves that control the multifidus muscle—a key stabilizer of the lumbar spine. By restoring muscle function and spinal stability, ReActiv8 seeks to provide lasting relief rather than mere symptom suppression.
The Science Behind Restorative Neurostimulation
The premise of restorative neurostimulation rests on the role of the multifidus muscle in spinal health. The multifidus is a deep muscle that runs along the vertebral column, providing segmental stability and supporting movement. In many patients with chronic low back pain, this muscle becomes inhibited—often due to nerve injury, pain, or arthrogenic (joint-related) dysfunction. This leads to a vicious cycle: reduced multifidus activity causes instability and further pain, reinforcing the inhibition.
Restorative neurostimulation aims to break this cycle. By electrically stimulating the medial branch of the dorsal ramus nerve (which innervates the multifidus), ReActiv8 activates the muscle, helping to restore its function, improve spinal stability, and ultimately reduce pain.
Indications and Patient Selection
ReActiv8 is specifically indicated for adults with chronic mechanical low back pain that has persisted for more than six months and has not responded to conventional medical management, including physical therapy, medications, and injections. Ideal candidates often exhibit impaired multifidus function, typically confirmed through clinical assessment or imaging.
Patients who may benefit from ReActiv8 usually:
- Experience disabling low back pain with or without leg symptoms
- Have not improved with surgery, or are not candidates for surgery
- Show evidence of multifidus dysfunction, such as reduced muscle contraction on ultrasound or MRI
- Have no major spinal deformities or widespread neuropathic pain
The ReActiv8 Device and Implant Procedure
The ReActiv8 system consists of an implantable pulse generator and two leads with electrodes that are positioned adjacent to the medial branch nerves at lumbar levels (usually L2–L4). The device is typically implanted under local or general anesthesia in a minimally invasive surgical procedure that takes about an hour.
The steps include:
- Identifying the target nerves using fluoroscopy or other imaging modalities
- Inserting the leads through small incisions and positioning them alongside the medial branches
- Connecting the leads to the pulse generator, which is implanted in a small pocket under the skin (usually in the lower back or flank)
- Programming the device to deliver individualized stimulation sessions
Following implantation, patients are instructed in using a handheld remote to activate the device, typically engaging in two 30-minute stimulation sessions per day.
How ReActiv8 Works
ReActiv8 delivers electrical pulses that stimulate the targeted nerves, causing the multifidus muscle to contract in a way that simulates natural movement. The therapy is not intended to provide immediate pain masking. Instead, with regular use, the stimulated contractions help “retrain” the multifidus, restoring its role in maintaining segmental spinal stability.
Over weeks to months, as the muscle regains strength and coordination, patients often report improvements in pain, function, and quality of life. Unlike conventional neuromodulation therapies, the goal is restoration, not just symptom control.
Clinical Evidence and Outcomes
ReActiv8 has been evaluated in several clinical trials, most notably the ReActiv8-B randomized controlled trial. Key findings from the published literature include:
- Significant Pain Reduction: Patients receiving restorative neurostimulation demonstrated clinically meaningful reductions in low back pain scores compared to baseline.
- Improved Function: Functional improvements were observed in activities of daily living and patient-reported outcome measures.
- Sustained Benefits: The improvements in pain and function persisted over long-term follow-up, with many patients maintaining or further improving their results at two years and beyond.
- Low Complication Rate: The procedure and device were generally well tolerated, with low rates of device-related complications and explants.
Notable Studies
– ReActiv8-B Study: A multicenter, randomized, sham-controlled trial established the efficacy and safety of the device. At one year, 57% of patients reported a significant reduction in pain, and 64% reported improvements in disability and quality of life.
– Long-Term Data: Studies up to four years post-implantation continue to demonstrate maintained or improved results, supporting the restorative concept.
Benefits and Advantages
ReActiv8 offers several advantages compared to standard treatments for chronic low back pain:
- Addresses Underlying Dysfunction: Rather than merely masking pain signals, the therapy targets the muscle dysfunction at the root of many cases of low back pain.
- Non-Destructive and Reversible: The procedure does not involve cutting or removing tissue and can be reversed if necessary.
- Minimal Side Effects: Side effects are generally mild and may include temporary soreness or minor discomfort at the implant site.
- Suitable for Patients Without Surgical Options: ReActiv8 can help those who are not candidates for, or wish to avoid, spinal surgery.
Potential Risks and Considerations
As with any implantable device, some risks exist, including infection, bleeding, lead migration, or device malfunction. However, these events are rare, and the majority of patients tolerate the procedure and device well.
Patients must also be committed to regular use of the device for optimal benefit, and some may experience initial muscle soreness as the multifidus is retrained.
The Patient Experience
Patient stories highlight the transformative impact of restorative neurostimulation. Many report regaining their ability to perform daily tasks, exercise, and even return to work after years of disability. The programming is typically painless, and most users can independently manage their therapy.
Follow-up visits are scheduled to monitor device function, adjust settings as needed, and support rehabilitation goals. Physical therapy may be recommended alongside ReActiv8 therapy to maximize outcomes.
Future Perspectives and Ongoing Research
Ongoing research is further defining the optimal patient selection criteria, stimulation parameters, and long-term outcomes of ReActiv8. As awareness grows and more clinicians are trained in the technique, the therapy’s reach is expected to expand, potentially benefiting a wider range of patients suffering from chronic mechanical low back pain.
Research is also underway to assess the benefits of combining ReActiv8 with other non-invasive treatments, rehabilitation protocols, or as a bridge to more definitive interventions.
Nursing Care of Patients with ReActiv8 (Restorative Neurostimulation)
Building upon these foundational principles, nursing care for patients with the ReActiv8 restorative neurostimulation system requires a blend of clinical vigilance, patient-centred education, and collaborative teamwork.
Assessment and Monitoring
Regular assessment of the implant site for signs of inflammation, infection, or device malfunction is crucial. Nurses should palpate the area gently, observe for erythema, swelling, or discharge, and document findings meticulously. Additionally, monitoring for systemic symptoms—such as fever, chills, or malaise—can provide early warning of potential complications.
Device Management and Troubleshooting
Patients benefit from hands-on guidance in using their device, including activating and deactivating stimulation cycles as prescribed. Nurses should verify the integrity of external components, such as remote controls or chargers, and ensure patients are comfortable with their use. In case of technical issues, initial troubleshooting may involve checking battery status, confirming device connectivity, or reviewing manufacturer-provided guides.
Pain and Symptom Control
Although ReActiv8 is designed to alleviate chronic low back pain, some patients may experience transient discomfort or unfamiliar sensations as they adapt to neurostimulation. Nurses should encourage open dialogue regarding pain levels, changes in sensation, or new symptoms, adjusting care plans in collaboration with prescribing clinicians as needed.
Patient Education and Empowerment
Education is ongoing: nurses reinforce the importance of device care, symptom tracking, and adherence to follow-up schedules. Empowering patients to participate actively in their care fosters self-efficacy and improves long-term outcomes. Tailored educational resources—such as written guides, instructional videos, or apps—may enhance understanding and ease anxiety.
Psychosocial Considerations
Living with an implant can evoke a range of emotions, including hope, apprehension, or concern about body image and lifestyle. Nurses can support patients by normalising these feelings, offering practical coping strategies, and facilitating access to counselling if needed. Encouraging engagement with support networks and peer communities helps patients feel connected and validated.
Interprofessional Communication
Coordinated care is the backbone of successful outcomes. Nurses liaise regularly with physicians, physiotherapists, and device manufacturer representatives, ensuring that all clinical changes, patient concerns, and technical queries are addressed promptly. Thorough documentation and timely updates fortify the safety net around the patient.
Long-Term Follow-Up
Ongoing surveillance for device efficacy and patient satisfaction is essential. Scheduled assessments may include physical examinations, pain scoring, and evaluation of functional or psychological improvements. Nurses are instrumental in identifying evolving needs and advocating for adjustments to care or device programming.
REFERENCES
- Garcia K, Wray JK, Kumar S. Spinal Cord Stimulation. https://www.ncbi.nlm.nih.gov/books/NBK553154/. 2023 Apr 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
- Gilligan C, Volschenk W, Russo M, et al. Long-Term Outcomes of Restorative Neurostimulation in Patients With Refractory Chronic Low Back Pain Secondary to Multifidus Dysfunction: Two-Year Results of the ReActiv8-B Pivotal Trial. https://pubmed.ncbi.nlm.nih.gov/35088722/. Neuromodulation. 2023 Jan;26(1):87-97.
- Gilligan C, Volschenk W, Russo M, ReActiv8-B Investigators. Long-Term Outcomes of Restorative Neurostimulation in Patients With Refractory Chronic Low Back Pain Secondary to Multifidus Dysfunction: Two-Year Results of the ReActiv8-B Pivotal Trial. Neuromodulation. 2023 Jan;26(1):87-97. doi: 10.1016/j.neurom.2021.10.011. Epub 2021 Dec 18. PMID: 35088722.
- International Neuromodulation Society. Restorative Neurostimulation. https://www.neuromodulation.com/restorative-neurostimulation. Last reviewed 8/2/2021.
- Gilligan C, Burnside D, Grant L, Yong RJ, Mullins PM, Schwab F, Mekhail N. ReActiv8 Stimulation Therapy vs. Optimal Medical Management: A Randomized Controlled Trial for the Treatment of Intractable Mechanical Chronic Low Back Pain (RESTORE Trial Protocol). Pain Ther. 2023 Apr;12(2):607-620.
- Mainstay Medical, ReActiv8® Restorative Neurostimulation™. ReActiv8 Patient Journey. https://lowbackpainrecovery.com/faq/. Last reviewed 7/8/2024.
- United States Food and Drug Administration (FDA). ReActiv8 Summary of Safety and Effectiveness. https://www.accessdata.fda.gov/cdrh_docs/pdf19/P190021B.pdf. Last updated 5/20/2020.
Stories are the threads that bind us; through them, we understand each other, grow, and heal.
JOHN NOORD
Connect with “Nurses Lab Editorial Team”
I hope you found this information helpful. Do you have any questions or comments? Kindly write in comments section. Subscribe the Blog with your email so you can stay updated on upcoming events and the latest articles.





