Glycogenesis is the biochemical process of forming glycogen from glucose. It occurs mainly in the liver and skeletal muscles, helping regulate blood sugar, store energy, and maintain metabolic balance. Essential in carbohydrate metabolism and energy management.
Introduction
Among the many biochemical pathways, glycogenesis—the process by which glucose is converted into glycogen for storage—is particularly relevant. Understanding glycogenesis is vital for nurses working with patients affected by metabolic disorders, diabetes, liver disease, and other conditions where energy balance and glucose regulation are crucial.
Overview of Glycogenesis
Glycogenesis is the metabolic pathway responsible for the synthesis of glycogen, a large, branched polysaccharide that serves as the primary storage form of glucose in the body. This process is essential for maintaining blood glucose homeostasis and providing a rapid energy source during periods of increased demand. For nurses, understanding glycogenesis offers insight into the management of conditions such as diabetes, hypoglycaemia, and glycogen storage diseases.
Basic Concepts
Definition of Glycogenesis
Glycogenesis is the biochemical process through which glucose molecules are linked together to form glycogen. This process primarily occurs in the liver and skeletal muscle cells. Glycogen acts as a readily mobilisable energy reserve, which can be broken down quickly when the body needs glucose, such as during fasting or intense physical activity.
The Role of Glycogenesis in Metabolism
Glucose is a critical energy source for many tissues, especially the brain and muscles. However, the body cannot store large amounts of free glucose due to its osmotic effects. Glycogenesis solves this problem by converting excess glucose into glycogen, a compact and osmotically inert storage form. This process:
- Prevents hyperglycaemia after meals by storing excess glucose
- Maintains blood glucose levels during fasting or between meals
- Provides a rapid source of glucose during physical activity
Biochemical Pathway: Step-by-Step Description of Glycogenesis
Glycogenesis involves several enzymatic steps that convert glucose to glycogen. Each step is tightly regulated and involves specific enzymes and cofactors. Below is a detailed, nurse-friendly explanation of the pathway.
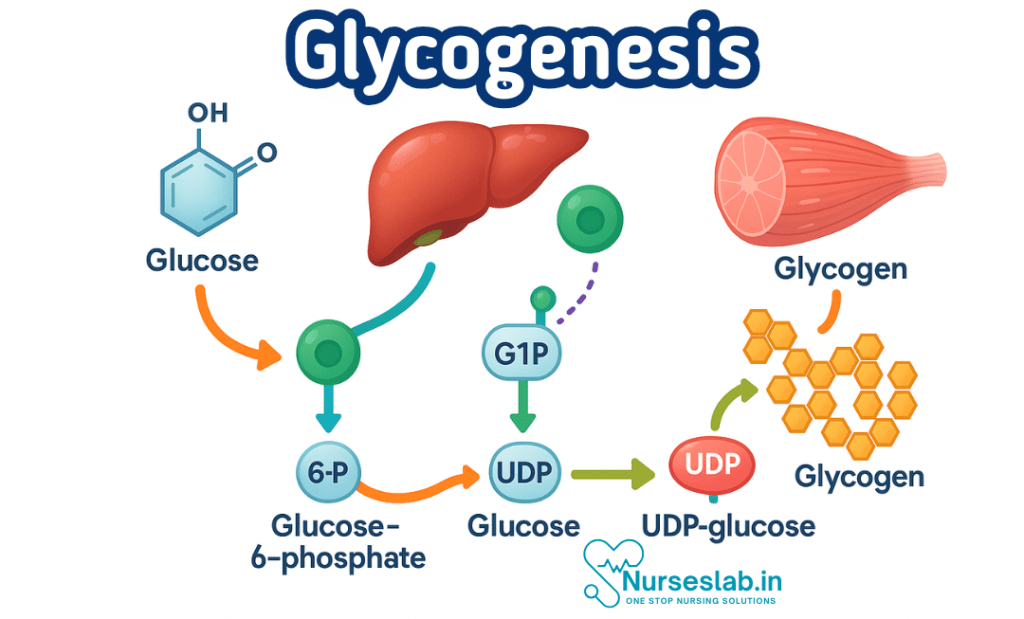
1. Glucose Uptake and Phosphorylation
The process begins when glucose enters the cell, mainly via facilitated diffusion through glucose transporter proteins (GLUTs). Once inside, glucose is rapidly phosphorylated by the enzyme hexokinase (in muscle) or glucokinase (in liver) to form glucose-6-phosphate (G6P). This phosphorylation traps glucose within the cell and marks it for metabolic processing.
2. Conversion to Glucose-1-Phosphate
The enzyme phosphoglucomutase then converts glucose-6-phosphate to glucose-1-phosphate (G1P). This is a reversible reaction and serves as a key step in glycogen metabolism.
3. Activation of Glucose: Formation of UDP-Glucose
Glucose-1-phosphate is not directly added to glycogen. Instead, it is activated by the enzyme UDP-glucose pyrophosphorylase, which attaches uridine diphosphate (UDP) to G1P, forming UDP-glucose. UDP-glucose is the immediate donor of glucose units for glycogen synthesis.
4. Glycogen Primer and Initiation
Glycogen synthesis requires a primer—a short chain of glucose molecules. In the absence of pre-existing glycogen, the protein glycogenin acts as a primer by autoglycosylating itself, providing the starting point for glycogen synthesis.
5. Elongation of Glycogen Chains
The enzyme glycogen synthase catalyses the addition of glucose units from UDP-glucose to the growing glycogen chain, forming α-1,4-glycosidic bonds. This is the main step in glycogen synthesis and is tightly regulated.
6. Branching of Glycogen
Glycogen is a highly branched molecule, which increases its solubility and allows for rapid mobilisation. The branching enzyme (amylo-1,4→1,6-transglucosidase) creates branches by transferring a segment of the glycogen chain and attaching it via an α-1,6-glycosidic bond. This branching is crucial for the efficient storage and retrieval of glucose.
Key Enzymes and Molecular Mechanisms
| Enzyme | Function | Location |
| Hexokinase/Glucokinase | Phosphorylates glucose to G6P | Muscle/Liver |
| Phosphoglucomutase | Converts G6P to G1P | Muscle/Liver |
| UDP-glucose pyrophosphorylase | Forms UDP-glucose from G1P | Muscle/Liver |
| Glycogen synthase | Adds glucose units to glycogen | Muscle/Liver |
| Branching enzyme | Introduces branches in glycogen | Muscle/Liver |
| Glycogenin | Acts as primer for new glycogen molecules | Muscle/Liver |
Regulation of Glycogenesis
Hormonal Regulation
Glycogenesis is primarily regulated by hormones—mainly insulin and glucagon—which signal the body’s energy status and coordinate glucose storage and mobilisation.
- Insulin: Secreted by the pancreas in response to elevated blood glucose, insulin stimulates glycogenesis by activating glycogen synthase and inhibiting glycogen breakdown. This is especially important after meals, helping to store excess glucose as glycogen.
- Glucagon: Released when blood glucose is low, glucagon inhibits glycogenesis and promotes glycogenolysis (glycogen breakdown), ensuring glucose is available for energy.
- Adrenaline (Epinephrine): In times of stress or increased energy demand, adrenaline inhibits glycogenesis in muscle, favouring glucose release for immediate use.
Metabolic Regulation
Several metabolic factors also regulate glycogenesis:
- Availability of glucose and ATP: High levels favour glycogen synthesis.
- Allosteric regulation: Glycogen synthase is allosterically activated by glucose-6-phosphate.
- Covalent modification: Enzymes involved in glycogenesis are regulated by phosphorylation and dephosphorylation, controlled by hormonal signals.
Factors Affecting Glycogenesis
Various physiological and pathological factors influence glycogenesis:
- Dietary intake: High carbohydrate meals increase glycogenesis.
- Exercise: Depletes glycogen stores, enhancing subsequent synthesis during recovery.
- Hormonal imbalances: Conditions such as diabetes mellitus affect insulin secretion and action, disrupting glycogenesis.
- Liver and muscle health: Diseases impacting these organs impair glycogen storage.
Clinical Relevance of Glycogenesis
Disorders Related to Glycogenesis
Understanding glycogenesis is crucial for the clinical management of several disorders:
- Glycogen Storage Diseases (GSDs): A group of inherited metabolic disorders caused by deficiencies in enzymes involved in glycogen synthesis or breakdown. Examples include:
- Type I (Von Gierke’s disease): Deficiency in glucose-6-phosphatase, leading to hypoglycaemia, hepatomegaly, and lactic acidosis.
- Type II (Pompe disease): Deficiency in lysosomal acid alpha-glucosidase, causing muscle weakness and cardiomegaly.
- Type V (McArdle’s disease): Deficiency in muscle phosphorylase, resulting in exercise intolerance and muscle cramps.
- Diabetes Mellitus: Impaired insulin secretion or action affects glycogenesis, contributing to hyperglycaemia and poor glucose control.
- Liver Diseases: Conditions such as hepatitis and cirrhosis disrupt glycogen metabolism, leading to abnormal glucose storage and release.
- Hypoglycaemia: Inadequate glycogen stores, often due to malnutrition or metabolic disease, can result in dangerously low blood glucose levels.
Implications for Patient Care
Nurses play a key role in recognising and managing the clinical consequences of impaired glycogenesis. This includes:
- Monitoring blood glucose in at-risk patients, such as those with diabetes or liver disease
- Recognising signs of hypoglycaemia (e.g., confusion, sweating, tachycardia) and hyperglycaemia (e.g., polyuria, fatigue, blurred vision)
- Educating patients and families about the importance of regular meals, adherence to medication, and monitoring for symptoms
- Supporting dietary interventions to optimise glycogen stores, especially in patients with increased metabolic demands (e.g., athletes, post-operative patients)
Nursing Practice: Monitoring, Interventions, and Patient Education
Monitoring Glycogen-Related Disorders
Nurses routinely monitor patients for signs and symptoms of metabolic disturbances. Key assessments include:
- Blood glucose monitoring using glucometers or laboratory analysis
- Observing for clinical features of hypoglycaemia or hyperglycaemia
- Assessing for hepatomegaly or muscle weakness in suspected glycogen storage diseases
- Reviewing dietary intake and physical activity levels
Nursing Interventions
Effective interventions are based on a clear understanding of glycogenesis and its disorders:
- Management of Hypoglycaemia: Prompt administration of fast-acting carbohydrates (e.g., glucose gel, fruit juice) and monitoring for response
- Support for Patients with Diabetes: Encouraging adherence to insulin regimens, dietary advice, and regular exercise to optimise glycogen stores
- Caring for Patients with Glycogen Storage Diseases: Collaborating with dietitians and metabolic specialists to provide specialised nutrition and supportive care
- Perioperative Care: Ensuring appropriate glucose management in surgical patients, especially those who are fasting or have metabolic disorders
Patient Education
Education is a critical component of nursing care. Nurses should provide clear, accessible information about:
- The role of glycogen in energy balance and why its regulation matters
- Recognising symptoms of metabolic disturbances and when to seek help
- Importance of regular meals and balanced nutrition
- Safe exercise practices, particularly for those with metabolic or muscular disorders
Summary and Key Takeaways
Glycogenesis is a vital biochemical process that enables the storage of glucose in the form of glycogen, maintaining energy balance and supporting physiological function. For nurses, understanding glycogenesis is essential for effective patient monitoring, early recognition of metabolic disturbances, and the delivery of high-quality care. Key points include:
- Glycogenesis converts excess glucose into glycogen, mainly in the liver and muscles
- The pathway involves several enzymes and is tightly regulated by hormonal and metabolic signals
- Disorders of glycogenesis can lead to serious clinical consequences, including hypoglycaemia, muscle weakness, and liver dysfunction
- Nurses play a crucial role in monitoring, intervention, and patient education related to glycogen metabolism
Continued learning and collaboration with multidisciplinary teams are recommended to enhance patient outcomes related to metabolic health.
REFERENCES
- Harbans Lal, Textbook of Applied Biochemistry and Nutrition& Dietetics 2nd Edition ,November 2024, CBS Publishers and Distributors, ISBN: 978-9394525757
- Suresh K Sharma, Textbook of Biochemistry and Biophysics for Nurses, 2nd Edition, September 2022, Jaypee Publishers, ISBN: 978-9354655760
- Peter J Kennelly, Harpers Illustrated Biochemistry Standard Edition, September 2022, McGraw Hill Lange Publishers, ISBN: 978-1264795673
- Denise R Ferrier, Ritu Singh, Lippincott Illustrated Reviews Biochemistry, Second Edition, June 2024, ISBN- 978-8197055973
- Yadav, Tapeshwar & Bhadeshwar, Sushma. (2022). Essential Textbook of Biochemistry for Nursing.
- Applied Sciences, Importance of Biochemistry for Nursing Practice, November 2, 2023, https://bns.institute/applied-sciences/importance-biochemistry-nursing-practice/
Stories are the threads that bind us; through them, we understand each other, grow, and heal.
JOHN NOORD
Connect with “Nurses Lab Editorial Team”
I hope you found this information helpful. Do you have any questions or comments? Kindly write in comments section. Subscribe the Blog with your email so you can stay updated on upcoming events and the latest articles.





