Protein digestion begins in the stomach with pepsin and continues in the small intestine with pancreatic enzymes. Amino acids are absorbed through intestinal villi into the bloodstream, supporting tissue repair, muscle growth, and essential metabolic functions.
Introduction
Proteins, complex macromolecules composed of amino acid chains, must be broken down into their constituent amino acids and small peptides before absorption. The journey from dietary protein to cellular amino acids involves a series of orchestrated biochemical reactions occurring in distinct segments of the gastrointestinal tract. This process is facilitated by specialised enzymes and regulated by hormonal and neural mechanisms, ensuring that the body’s protein needs are met efficiently and safely.
Structure and Function of Proteins
Amino Acids: The Building Blocks
Proteins are polymers of amino acids, organic compounds containing an amino group (-NH2), a carboxyl group (-COOH), and a unique side chain (R group). There are twenty standard amino acids, classified as essential (cannot be synthesised by the body and must be obtained from the diet) and non-essential (can be synthesised internally). Amino acids link via peptide bonds, forming linear chains that fold into complex structures.
Protein Structure
- Primary Structure: Linear sequence of amino acids.
- Secondary Structure: Localised folding into α-helices and β-sheets, stabilised by hydrogen bonds.
- Tertiary Structure: Three-dimensional folding driven by interactions among side chains.
- Quaternary Structure: Association of multiple polypeptide chains to form functional proteins.
Biological Roles of Proteins
Proteins serve diverse functions: enzymes catalyse biochemical reactions, structural proteins maintain cell and tissue integrity, transport proteins carry molecules across membranes, hormones regulate physiological processes, and antibodies provide immune defence. The body’s protein requirements are met through dietary intake, digestion, and absorption, making these processes vital for health.
Overview of Digestive System
Organs Involved in Protein Digestion
- Mouth: Initial mechanical processing of food.
- Stomach: Chemical breakdown of proteins begins.
- Small Intestine: Major site for enzymatic digestion and absorption.
- Pancreas: Secretes digestive enzymes.
- Liver: Produces bile, indirectly supporting digestion.
General Process of Digestion
Digestion is a coordinated process involving mechanical breakdown (chewing, mixing) and chemical hydrolysis (enzymatic cleavage). Proteins are unique in requiring multiple enzymatic steps, as peptide bonds are resistant to non-specific hydrolysis.
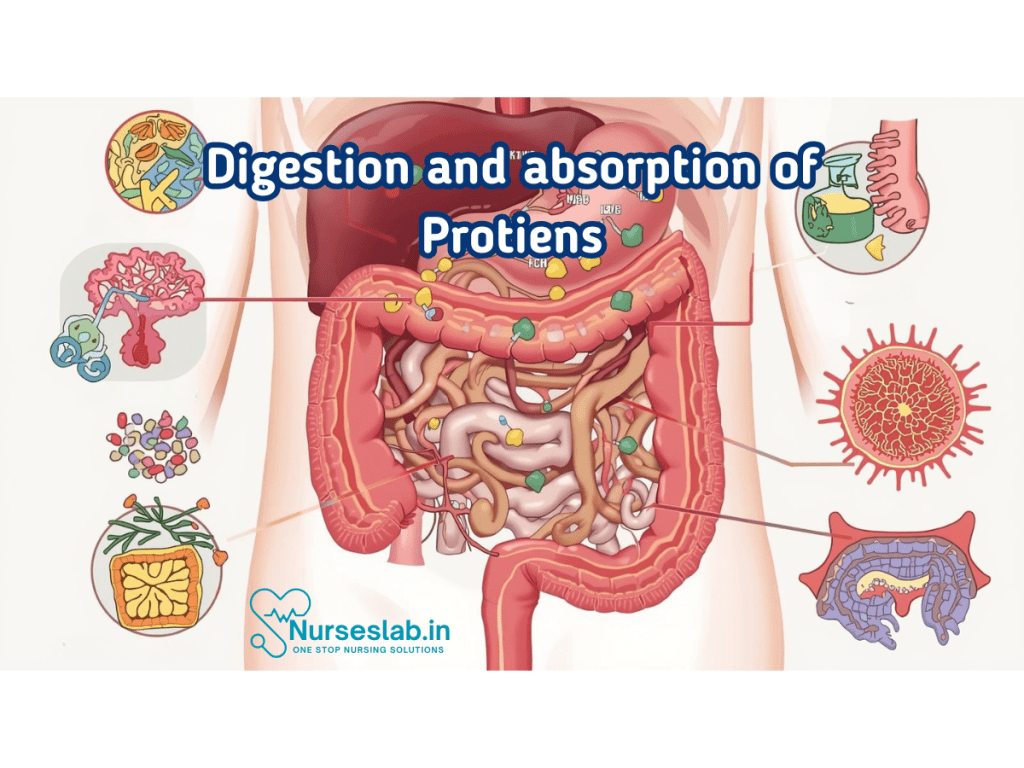
Digestion of Proteins
Oral Cavity
Protein digestion does not begin in the oral cavity, as saliva lacks proteolytic enzymes. However, mastication breaks food into smaller pieces, increasing the surface area for subsequent enzyme action.
Stomach: Pepsin and Gastric Acid
The stomach marks the onset of protein digestion. Gastric glands secrete hydrochloric acid (HCl), which denatures proteins, exposing peptide bonds. Chief cells release pepsinogen, an inactive precursor (zymogen), which is converted to active pepsin by acidic pH. Pepsin cleaves peptide bonds, especially those adjacent to aromatic amino acids, producing shorter polypeptides and some free amino acids.
- Role of HCl: Denatures protein structure, activates pepsinogen.
- Pepsin: Endopeptidase, initiates protein hydrolysis.
Protein digestion in the stomach is incomplete; most proteins exit as large polypeptides.
Small Intestine: Pancreatic Enzymes and Brush Border Enzymes
The partially digested proteins enter the duodenum, where the pancreas secretes a suite of potent proteases:
- Trypsin: Cleaves peptide bonds at the carboxyl side of lysine and arginine.
- Chymotrypsin: Targets bonds adjacent to aromatic amino acids.
- Carboxypeptidases: Remove terminal amino acids from the carboxyl end.
- Elastase: Acts on small neutral amino acids.
These enzymes are secreted as inactive zymogens (trypsinogen, chymotrypsinogen, procarboxypeptidase) and activated in the intestinal lumen. Enteropeptidase, an enzyme produced by the duodenal mucosa, initiates this cascade by activating trypsinogen to trypsin, which then activates other proteases.
Further digestion occurs at the brush border of enterocytes, where peptidases complete the breakdown into dipeptides, tripeptides, and free amino acids.

Absorption of Proteins
Mechanisms of Amino Acid and Peptide Absorption
Absorption of amino acids and small peptides occurs primarily in the jejunum and ileum. The process involves specialised transporters located on the apical membrane of enterocytes.
- Amino Acid Transporters: Each transporter is specific for a class of amino acids (neutral, basic, acidic, imino).
- Dipeptide and Tripeptide Transporters: Peptide transporter 1 (PEPT1) facilitates uptake of small peptides, which are later hydrolysed to amino acids within the enterocyte.
Transport is driven by sodium gradients (secondary active transport), maintained by the Na+/K+ ATPase pump on the basolateral membrane. Amino acids and peptides are then released into the portal circulation for delivery to the liver.
Role of Enterocytes
Enterocytes, the absorptive cells lining the small intestine, play a central role in protein absorption. They possess brush border enzymes, facilitate transport, and regulate the passage of nutrients into the bloodstream. Some amino acids are metabolised within enterocytes for local energy needs.
Transporters and Pathways
| Transporter | Substrate | Mechanism |
| PEPT1 | Dipeptides, Tripeptides | H<sup>+</sup>-coupled transport |
| SLC6 family | Neutral, Basic, Acidic amino acids | Na<sup>+</sup>-coupled transport |
| SLC7 family | Cationic amino acids | Facilitated diffusion |
Regulation of Digestion and Absorption
Hormonal Control
- Gastrin: Stimulates gastric acid and pepsinogen secretion.
- Cholecystokinin (CCK): Triggers pancreatic enzyme release.
- Secretin: Promotes bicarbonate secretion to neutralise gastric acid.
These hormones are released in response to the presence of proteins and peptides in the stomach and duodenum, ensuring that enzymes and optimal pH are available for digestion.
Neural Control and Feedback Mechanisms
The enteric nervous system coordinates motility and secretion. Vagal stimulation enhances enzyme release, while feedback inhibition prevents overproduction. For example, high levels of free amino acids in the lumen can down-regulate further enzyme secretion.
Clinical Implications
Disorders of Protein Digestion and Absorption
- Pancreatic Insufficiency: Deficient enzyme secretion leads to incomplete protein digestion, causing steatorrhoea and malnutrition.
- Celiac Disease: Immune-mediated damage to enterocytes impairs absorption, leading to diarrhoea, weight loss, and nutrient deficiencies.
- Tropical Sprue: Infectious or idiopathic enteropathy affecting absorption.
- Congenital Disorders: Hartnup disease (defective neutral amino acid transport), cystinuria (impaired cystine reabsorption).
Malabsorption Syndromes
Malabsorption syndromes encompass conditions where nutrients, including proteins, are inadequately absorbed. Symptoms include chronic diarrhoea, weight loss, oedema, and anaemia. Diagnosis involves stool analysis, blood tests, and sometimes endoscopic biopsy.
Diagnostic Approaches
- Stool Examination: Detects undigested proteins.
- Serum Amino Acid Levels: Assesses absorption efficiency.
- Imaging and Biopsy: Identifies structural or functional defects in the intestine.
Nursing Perspectives
Assessment
Nurses play a pivotal role in recognising signs of protein malnutrition, such as muscle wasting, oedema, poor wound healing, and altered mental status. Comprehensive nutritional assessment includes dietary history, physical examination, and laboratory investigations.
Patient Education
Educating patients about the importance of protein in the diet, sources of high-quality proteins (e.g., eggs, dairy, pulses, fish, lean meats), and the consequences of deficiency is essential. Nurses should tailor advice to individual needs, cultural preferences, and economic constraints.
Nutritional Support
For patients unable to meet protein requirements orally, nurses coordinate enteral or parenteral nutrition, monitor for complications, and collaborate with dietitians. Special attention is required for vulnerable groups, such as children, elderly, and post-operative patients.
Monitoring and Interventions
- Regular monitoring of weight, serum albumin, and nitrogen balance.
- Early identification and management of malnutrition.
- Implementation of dietary modifications as per clinical guidelines.
- Interdisciplinary collaboration for optimum patient outcomes.
REFERENCES
- Harbans Lal, Textbook of Applied Biochemistry and Nutrition& Dietetics 2nd Edition ,November 2024, CBS Publishers and Distributors, ISBN: 978-9394525757
- Suresh K Sharma, Textbook of Biochemistry and Biophysics for Nurses, 2nd Edition, September 2022, Jaypee Publishers, ISBN: 978-9354655760
- Peter J Kennelly, Harpers Illustrated Biochemistry Standard Edition, September 2022, McGraw Hill Lange Publishers, ISBN: 978-1264795673
- Denise R Ferrier, Ritu Singh, Lippincott Illustrated Reviews Biochemistry, Second Edition, June 2024, ISBN- 978-8197055973
- Yadav, Tapeshwar & Bhadeshwar, Sushma. (2022). Essential Textbook of Biochemistry for Nursing.
- Applied Sciences, Importance of Biochemistry for Nursing Practice, November 2, 2023, https://bns.institute/applied-sciences/importance-biochemistry-nursing-practice/
Stories are the threads that bind us; through them, we understand each other, grow, and heal.
JOHN NOORD
Connect with “Nurses Lab Editorial Team”
I hope you found this information helpful. Do you have any questions or comments? Kindly write in comments section. Subscribe the Blog with your email so you can stay updated on upcoming events and the latest articles.





